Properties of Water Experiments


Basics on the topic Properties of Water Experiments
Want to do some water experiments at home? Learn three different water experiments you can do at home in this video!
Transcript Properties of Water Experiments
Uma is already excited to see our video on properties of water experiments. How will this work? In this video you will be shown three experiments. Each of which can be replicated at home in three simple steps. First, you will learn what materials you need and how to set up the experiment. Then, like a scientist, you predict what will happen. And then the experiment can be carried out. We will hide the result of all the experiments until the end of the video. Let's get started on the first experiment; Uma is waiting! For this experiment you need a glass, a dry-erase marker, and water. Fill the glass halfway with water, mark the water level with a line, and place it somewhere quiet where it will not be disturbed for the next few weeks. It should not be placed by a window or on a heater. Then you can write down your prediction, and you should also check the amount of water in the jar every two to three days and mark the new water level with the dry-erase marker. For your prediction, answer the questions: What will you conclude from the mark? Why does this happen? Press pause briefly if you need the time to write. This experiment is expected to last two to three weeks. Until then, you can conduct your second experiment. For this you need a refrigerator, a glass of water, and a warm day. Put the glass with water in the refrigerator for two to three hours. Now you have time to think: What will happen when you take the glass of water out of the refrigerator? Why do you think this will happen? Write down your prediction. Is it written down? Then you can begin the experiment by removing the glass of water from the refrigerator after two to three hours and observing it. Or do you still want to do something else? We'll show you the third experiment now: For this, you will need two identical glasses, water, ice cubes, and a scale. Now comes the tricky part: For the experiment, you will put one hundred grams of water into the one glass, and one hundred grams of ice in the other glass. Then simply place both on a table. Make a prediction: Which glass has more liquid after the ice has melted? Do you know why? You can find out! You are now familiar with all three experiments! Attention attention: The results of your experiments are hidden behind this curtain! In the first experiment, you made a line every two to three days at the height where the water level is. You'll notice that it continues to get lower. This is due to the fact that the water evaporates over time. As a result, it turns into gas and water vapor and gradually disappears from the glass. In the second experiment, you took the glass of water out of the refrigerator. The glass of water from the refrigerator is SO cold that moisture in the air from the surrounding warmer air condenses on the outside of the glass. Water vapor then becomes water on the cold glass. This causes water droplets to form on the outside of your glass. In the third experiment, you had one hundred grams of water in one glass, and one hundred grams of ice in the other glass. After the ice had melted, both glasses should have equal amounts of water. Exciting, isn't it? What else did you observe during your experiments? Feel free to write it in the comments and share with each other!
Properties of Water Experiments exercise
-
Explain what will happen.
HintsWhy is it important that the water glass is not on a heater or near an open window?
If the water level went up, where did the extra water come from?
The water will gradually evaporate. What does evaporate mean? Is this a slow process or a fast one?
Solution- The water level will go down slightly every two or three days. You can see this by marking the new level with a permanent marker every two or three days.
- The reason the water level goes down is due to evaporation.
-
What will happen?
HintsThe glass should be in the refrigerator for two to three hours. After you take it out into a warm room, how will the glass feel?
The glass should be in the refrigerator, NOT the freezer.
Condensation occurs when water vapor in warm air hits a cold surface and turns back into liquid form.
There are two correct sentences, and three false.
SolutionLIKELY RESULTS
- The glass and water will be very cold.
- The glass will have water droplets on the outside after a few minutes.
- The glass and water will NOT be at room temperature after two hours in the refrigerator, because this experiment needs to be done on a warm day.
- The glass will NOT be dry on the outside because of the temperature difference between the water in the glass and the air around it.
- The water in the glass will NOT be frozen, because the glass should go in the refrigerator and NOT the freezer.
-
What will happen?
HintsThe only way the glass with 100 grams of ice can be empty, is if the ice turns into gas and evaporates. Is it hot enough for the ice to melt, then boil, and turn into steam?
Although the ice has a larger volume than the liquid water (takes up more space), they both have exactly the same weight.
There is only one correct choice.
SolutionThe glass with the original water, and the glass with the melted ice, will have the same amount of liquid after the ice melts. This is because even though the ice cubes take up more space than the liquid water, the weight is the same. Therefore, the amount of water is the same, even if it is in the form of ice.
-
Explain what happened.
HintsThe water glass should not be near an open window or a heat source.
The water glass should be in a quiet place where no one and nothing disturbs it.
There are two correct sentences that should be highlighted, and three false ones.
Solution- The water turned into water vapor over time.
- Water vapor evaporated out of the glass, making it more empty.
-
Label the different phases of water.
HintsOrange juice, milk, and anything you can pour is a liquid.
Cream is a liquid and ice cream is a solid.
If you try to touch a gas or vapor, you may not even feel it, and your hand will go straight through.
SolutionThe different phases of water are:
- Solid as ice
- Liquid as water
- Vapor or gas as a cloud
-
Explain the result.
HintsWater has three forms:
- Solid (ice)
- Liquid (water)
- Vapor or gas (steam, clouds, mist, and so forth)
One of the choices is used twice in two separate sentences.
When water turns from a solid to a liquid, this is melting. When water turns from a liquid to a gas, this is evaporation, and when water turns from a gas to a liquid, this is condensation.
Solution1) The glass became very cold in the refrigerator. When you took the glass out, there was a difference in temperature between the glass and the warm air.
2) There is always water vapor in the air on Earth. This stays in gas form in the warm air.
3) When the water vapor in the warm air hits the cold glass, it condenses and turns into liquid form.
4) The water droplets on the outside of the glass are actually condensed water vapor from the air outside the glass!




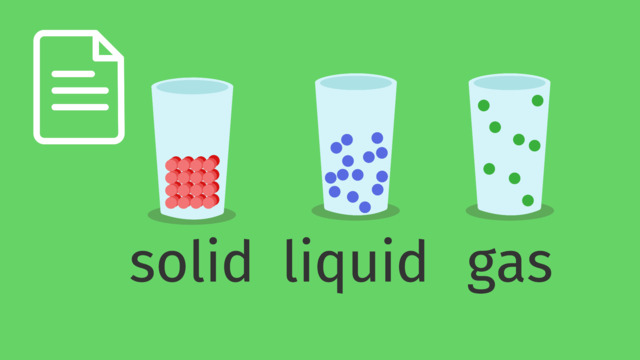










(✿◡‿◡)
amazing <3
Cool 😎 :)
Nice :)
I love this song